作者简介: 夏冬梅(1989-),女,重庆人,硕士研究生,研究方向为恶性肿瘤的放化疗。
目的 系统评价FcγRⅡa和FcγRⅢa 基因多态性与西妥昔单抗为基础的药物在治疗转移性结直肠癌疗效的相关性。方法 通过检索数据库,纳入2016年2月前所有符合纳入标准的有关FcγRⅡa和(或)FcγRⅢa基因多态性与西妥昔单抗在转移性结直肠癌中疗效的研究,采用Stata 11.0 软件进行Meta分析,用比值比及95%可信区间评价效应强度。结果 共12篇文章符合纳入标准,累计1 016个病例。Meta分析的合并结果显示,FcγRⅡa和FcγRⅢa基因多态性与西妥昔单抗为基础的药物治疗在转移性结直肠癌患者的疗效无相关性。亚组分析结果显示,FcγRⅡa-H131R位点的突变型可增加西妥昔单抗在KRAS突变的转移性结直肠癌患者的疗效(RR vs. HH: 比值比2.395,95%可信区间1.522~3.771, I2=0.000; R vs. H: 比值比2.072,95%可信区间1.617~2.654, I2=0.457; HR+RR vs. HH: 比值比 3.067,95%可信区间1.582~5.944, I2=0.692; HR vs. HH: 比值比11.222,95%可信区间1.324~95.106, I2=0.769);FcγRⅢa-V158F的突变型可增加亚洲人群对西妥昔单抗的疗效(FF vs. VF+VV: 比值比1.534,95%可信区间1.138~2.069, I2=0.000; FF/VF: 比值比1.398,95%可信区间 1.040~1.879, I2=0.000)。结论 FcγRⅡa和FcγRⅢa基因多态性与西妥昔单抗为基础的药物治疗在转移性结直肠癌中的疗效相关性可能存在种族差异和基因与基因的交互作用,需更多大样本高质量研究加以揭示。
Objective The purpose of this study is to evaluate the associations between FcγRⅡa and FcγRⅢa gene polymorphisms and Cetuximab-based treatment outcomes in metastatic colorectal cancer patients.Methods Eligible studies were searched from databases. Relative ratios (RR) with the corresponding 95% confidence interval (95%CI) were conducted. Statistical analysis was used with Stata 11.0.Results 12 articles were included, totally 1 016 cases. Pooled results indicated that no significant associations were found in FcγRⅡa and FcγRⅢa gene polymorphisms and Cetuximab-based treatment outcomes in metastatic colorectal cancer patients. In subgroup analysis, both KRAS and FcγRⅡa-H131R gene polymorphisms were significant association with Cetuximab-based treatment outcomes(RR vs. HH: RR=2.395,95%CI 1.522~3.771, I2=0.000; R vs. H: RR=2.072,95%CI 1.617~2.654, I2=0.457; HR+RR vs. HH: RR=3.067,95%CI 1.582~5.944, I2=0.692; HR vs. HH: RR=11.222,95%CI 1.324~95.106, I2=0.769), while FcγRⅢa-V158F gene polymorphism was seemed to be associated with treatment outcomes in Asian(FF vs. VF+VV: RR=1.534,95%CI 1.138~2.069, I2=0.000; FF/VF: RR=1.398,95%CI 1.040~1.879, I2=0.000).Conclusion FcγRⅡa and FcγRⅢa gene polymorphisms may be associated with Cetuximab-based treatment outcomes in metastatic colorectal cancer. Because racial differences and gene-to-gene interactions may influence such associations, more high-quality and large-scale studies are urgently needed.
结直肠癌有着很高的发病率和死亡率, 很多病例在确诊时就已出现转移[1]。虽然近年来新的化疗制剂及靶向药物使中位生存时间从12个月提高到2年, 但其死亡率仍可占每年新诊断病例的40%~50%, 居世界癌症死亡率的第二位[2, 3]。
西妥昔单抗是作用于表皮生长因子的一种靶向药物, 其单用或配合化疗药在治疗转移性结直肠癌中显示着可观的疗效[4, 5, 6]。KRAS基因状态在目前被认为与西妥昔单抗治疗转移性结直肠癌(metastatic colorectal cancer, mCRC)的疗效有关, 但其确切关系仍未得到确证[7, 8, 9]。西妥昔单抗可通过其Fc段与免疫细胞的免疫球蛋白G的 Fc片段受体(Fcγ R)结合而发挥抗体介导的细胞毒作用(ADCC)[10]。Fcγ RⅡ a 的多态性导致氨基酸131位点的组氨酸(H)突变为精氨酸(R), 而Fcγ RⅢ a的多态性导致氨基酸158位点的缬氨酸(V)突变为苯丙氨酸(F), 从而影响IgG的亲和力[11]。目前, 已有许多研究探讨该位点基因多态性与西妥昔单抗治疗mCRC的疗效关系, 但结果不一致, 主要原因可能为各研究纳入的样本量小, 混杂因素多且无法避免[12, 13, 14]。因此, 本研究拟对所有关于Fcγ RⅡ a和(或)Fcγ RⅢ a基因多态性与西妥昔单抗为基础的药物治疗mCRC的疗效关系的研究进行Meta分析, 以期充分揭示二者的关系, 为临床上西妥昔单抗在mCRC的利用上实现价值更优化提供依据。
采用主题词检索, “ metastatic colorectal cancer” “ Fcγ RⅡ a or FCGRⅡ A” “ Fcγ RⅢ a or FCGRⅢ A” “ polymorphism” “ Cetuximab” 。检索数据库为PubMed、EMBASE、Cochrane 图书馆、中国知网(CNKI)、中国生物医学文献数据库(CBM)、万方、重庆维普等数据库, 检索未设语言限制。截止时间为2016年2月。
(1)探究Fcγ RⅡ a 或Fcγ RⅢ a相关位点基因多态性与西妥昔单抗治疗mCRC疗效相关性的文章; (2)能提取基因频数分布和疗效的目标数据; (3)若研究重复, 保留样本量大及最新的研究。
(1)非该基因位点多态性与西妥昔单抗治疗mCRC疗效相关性的研究; (2)若研究人群重复, 剔除样本量小及旧的文章; (3)未把疗效相关指标作为研究指标; (4)文章中无可提取目标数据, 经联系作者未果的文章。
所有数据由两个研究者分别提取, 表格为经预提取后确定的标准数据表格形式。数据包括如下内容:第一作者, 出版年份, 地区, Fcγ RⅡ a 和Fcγ RⅢ a位点遗传模型的病例数以及治疗有效病例数等。
本文疗效“ 有效” 是指肿瘤客观有效(objective response, OR), 包括完全缓解(complete response, CR)和部分缓解(partial response, PR), 无效包括疾病稳定(stable disease, SD)和疾病进展(disease progression, PD)。根据WHO规定:CR为所有可测量病灶完全消失且持续4周; PR 为病灶缩小50%以上, 至少持续4周; SD为病灶减小小于50%, 或增大小于25%, 至少持续4周, 且无新病灶出现; PD为病灶增大25%, 或出现新发病灶。
所有资料均在Stata11.0 软件上完成(Stata Corporation, College Station, TX, USA)。采用比值比 (odds ratio, RR)及其95%可信区间(confidence interval, CI)评价效应强度, 采用χ 2和I2值评定异质性的大小(P< 0.05, 存在异质性; I2> 0.50, 异质性大), 若不存在异质性, 则采用固定效应模型(Peto法)进行合并, 反之, 则选用随机效应模型(D-L法), 并通过亚组分析探讨异质性的来源。采用Begg’ s检验和 Egger’ s漏斗图评价发表偏倚。
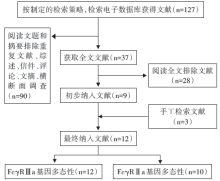
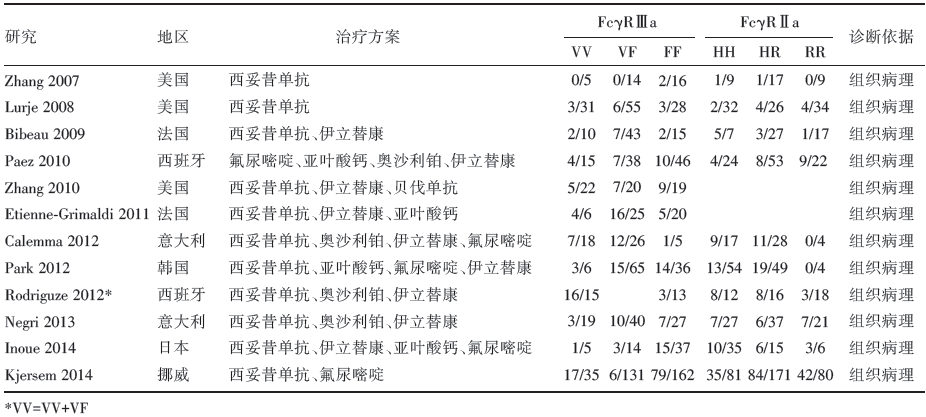
输入检索词, 初筛出127篇文章, 通过阅读题目和摘要, 筛选出37篇文章, 通过阅读全文及参考文献进行再次筛选, 最终纳入12篇文章[12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23]。其中关于Fcγ RⅡ a基因的有10篇[12, 13, 14, 15, 16, 17, 18, 19, 20, 23], 关于Fcγ RⅢ a基因的有12篇[12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23] , 具体检索流程见图1。其中欧洲人群10篇, 亚洲人群2篇。具体信息见表1。
| 表1 纳入文献基本特征及Fcγ RⅡ a 和Fcγ RⅢ a 基因型分布 |
2.2.1 关于Fcγ RⅡ a-H131R的结果
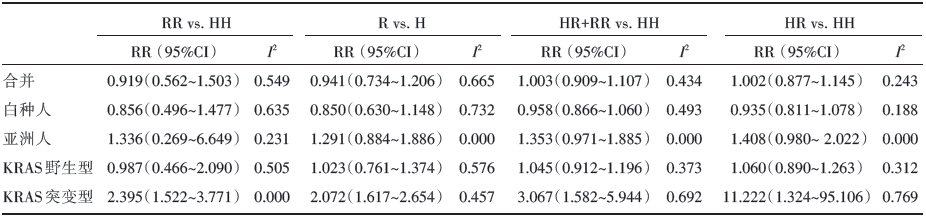
由于纯合子模型(RR vs. HH)和等位模型(R vs. H)存在异质性, 我们采用随机效应模型来进行合并, 共显模型(HR vs. HH)和显性模型(HR+RR vs. HH)则采用固定效应模型。各遗传模型的合并结果提示Fcγ RⅡ a -H131R的多态性与西妥昔单抗在转移性结直肠癌的疗效无相关性(见表2)。在亚组分析中, 以人种为基础分组的结果显示在亚洲人和高加索人中仍未显示任何的疗效差异, 而在以KRAS基因突变情况分组的亚组分析结果却提示Fcγ RⅡ a-H131R位点的突变型可增加西妥昔单抗在KRAS突变的mCRC患者的疗效(RR vs. HH: RR=2.395, 95%CI 1.522~3.771, I2=0.000; R vs. H:RR=2.072, 95%CI 1.617~2.654, I2=0.457; HR+RR vs. HH: RR=3.067, 95%CI 1.582~5.944, I2=0.692; HR vs. HH: RR=11.222, 95%CI 1.324~95.106, I2=0.769)。
| 表2 Fcγ RⅡ a 基因多态性与西妥昔单抗治疗转移性结直肠癌疗效相关性的Meta 分析结果 |
2.2.2 关于Fcγ RⅢ a-V158F的结果
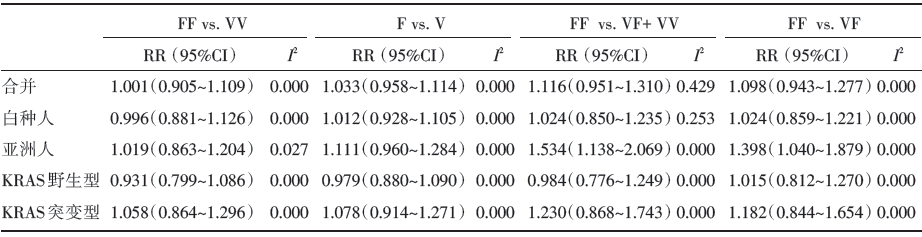
由于不存在统计学意义的异质性(P> 0.05), 所以采用固定效应模型。合并分析结果提示Fcγ RⅢ a-V158F的多态性与西妥昔单抗在mCRC的疗效无相关性(见表3)。在亚组分析中, 亚洲人组却显示Fcγ RⅢ a-V158F的突变型可增加西妥昔单抗的疗效(FF vs. VF+VV: RR=1.534, 95%CI 1.138~2.069, I2=0.000; FF vs. VF: RR=1.398, 95%CI 1.040~1.879, I2=0.000), 而以KRAS基因突变情况分组的亚组分析结果显示KRAS基因状态不影响该药物对mCRC的治疗效果。
| 表3 Fcγ RⅢ a 基因多态性与西妥昔单抗治疗mCRC 疗效相关性的Meta 分析结果 |
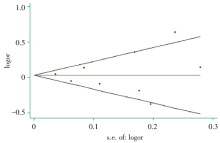
用Begg’ s 检验 和 Egger’ s漏斗图法评估纳入文献的发表偏倚, 绘制的漏斗图基本对称, Begg’ s检验的P> 0.05, 提示不存在明显的发表偏倚, 总体结果可靠, 见图2。
通过对纳入的各研究以逐一排除的方式进行敏感性分析, 结果显示各研究对合并RR值均不产生重大影响, 提示研究结果可靠。
西妥昔单抗可通过与癌细胞表面的EGFR特定区域竞争性结合, 阻断其胞内信号传导, 减少基质金属蛋白酶、AKT蛋白及血管内皮生长因子的生成, 进而发挥抗肿瘤作用[24]。同时, 其人源化成分可与免疫效应细胞(如NK细胞、巨噬细胞)上的IgG的Fc片段受体结合, 从而发挥ADCC作用, 因此认为Fcγ RⅡ a和Fcγ RⅢ a的突变将直接通过调控ADCC影响药物发挥抗肿瘤作用, 如有三项研究提出该基因的突变可降低疾病的反应率[13, 18, 21]。而本研究结果显示Fcγ RⅡ a和Fcγ RⅢ a的多态性与西妥昔单抗治疗mCRC的疗效无相关性。该结论与Paez等[14, 15, 16, 17, 23, 25]的研究相似, 认为在离体模型中ADCC作用是显著的, 但是在晚期肿瘤尤其是转移性肿瘤中, 常常发现NK细胞功能紊乱而引起ADCC作用显著受损[26]。另外, 肿瘤微环境可提高免疫细胞的扩增, 进而下调西妥昔单抗调节的ADCC的功能[27]。在以人种分组的亚组分析中, 发现Fcγ RⅢ a的突变型在亚洲人群中可获益而与白种人无关, 这与Inoue等[12]的结论一致, 提示基因型在人种的分布或表达情况的差异也可能是影响西妥昔单抗在mCRC中疗效的重要影响因素, 但由于样本量少(248例), 还需进一步研究。
KRAS的突变状态一度成为结直肠癌患者是否选用靶向药物的参考标志, 但越来越多研究证据的累积认为, KRAS的突变并不标志肿瘤细胞对ADCC作用的抵抗, 更重要的是, 作用于EGFR的Fc段的单抗能通过一些免疫机制诱导KRAS突变细胞的溶解[9, 28]。本研究根据KRAS的突变状态进行亚组分析, 发现Fcγ RⅡ a基因突变在KRAS突变患者中有更高的反应率。因此, 提示两者基因之间的交互作用可能也是影响靶向药物疗效的独立危险因素之一, 但由于样本量少, 该假设应谨慎对待, 需要进一步加以证实。
本研究是第一个对Fcγ RⅡ a和Fcγ RⅢ a 基因多态性与西妥昔单抗为基础的药物在mCRC治疗疗效关系进行的Meta分析, 通过对目前几乎所有研究进行数据整合, 使得研究结果较小样本的单个研究更可靠, 也更有说服力。但仍然有如下缺点:异质性的存在, 通过亚组分析, KRAS的突变状态是异质性的一个来源, 而基因型的分布, 基因与基因、基因与环境的交互作用皆可为异质性的来源, 但由于文献提供的资料不齐全, 我们不能对其进行具体来源的探讨。此外, 在所纳入文章中, 仅Zhang和Lurje 等[20, 23]的研究采用西妥昔单抗单药治疗, 其余文章均联合化疗治疗, 故不同化疗药与西妥昔单抗的相互作用不能否定会是影响研究结论的一个因素。最后, 虽然本文通过软件检验没有发表偏倚, 但我们仍不能排除发表偏倚的存在。需要更多大样本、高质量的研究来进一步揭示该基因多态性与西妥昔单抗在mCRC的疗效关系。
Fcγ RⅡ a和Fcγ RⅢ a基因多态性与西妥昔单抗为基础的药物在mCRC中的疗效相关性可能存在种族差异和基因与基因的交互作用, 需更多大样本、高质量研究加以揭示。
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|