作者简介:
廖一夫(1986-),男,广东梅州人,主治医师,医学硕士,主要研究方向为神经病学/肿瘤学。
目的 探讨RNA结合蛋白异质性胞核核糖核蛋白H1(heterogeneous nuclear ribonucleoprotein H1,hnRNPH1)在胶质瘤中的表达情况及其临床意义。方法 选取2000年1月至2012年12月于广东省人民医院收治进行手术的胶质瘤患者311例。取胶质瘤组织作为研究样本,取癌旁正常脑组织作为对照样本。利用大数据生物信息学数据库分析hnRNPH1的泛癌种表达量,采用免疫组织化学(immunohistochemictry,IHC)方法检测组织芯片中hnRNPH1在胶质瘤组织及癌旁正常组织中的表达量,卡方检验法分析胶质瘤组织中hnRNPH1的表达水平与患者临床病理参数之间的关系。单因素和多因素回归分析hnRNPH1表达水平和临床病理参数对胶质瘤患者生存的预测作用。Kaplan-Meier曲线法分析hnRNPH1表达水平与肿瘤患者生存期的关系。结果 分析癌症和肿瘤基因图谱(Cancer Genome Atlas,TCGA)数据库显示hnRNPH1在泛癌种组织中普遍高表达,与正常组织相比,hnRNPH1在胶质母细胞瘤组织中具有更高的表达水平,且随着患者年龄增加,hnRNPH1的表达水平逐渐增加。采用IHC检测胶质瘤组织芯片hnRNPH1的表达,结果显示hnRNPH1表达水平与胶质瘤级别正相关。生存分析结果显示,高表达hnRNPH1的患者总生存期短。hnRNPH1的表达与患者年龄、性别、肿瘤定位无关( P>0.05),与肿瘤类型和肿瘤级别相关( P<0.05);hnRNPH1的表达水平、患者年龄及肿瘤级别是预后的独立预测因素( P<0.05)。结论 胶质瘤组织中hnRNPH1的表达水平明显上调,与肿瘤患者的不良预后密切相关,可能参与胶质瘤的发生发展过程。
Objective To investigate the expression of RNA-binding protein heterogeneous nuclear ribonucleoprotein H (hnRNPH1) in glioma and its clinical significance.Methods From January 2000 to December 2012, 311 patients with glioma underwent surgery in Guangdong Provincial People's Hospital were selected. The glioma tissue was taken as the study sample, while the normal brain tissue adjacent to the cancer was taken as the control sample. hnRNPH1 protein extracted from glioma tissues and normal tissues adjacent to cancer was assessed by immunohistochemistry (IHC) in tissue microarray. hnRNPH1 protein expression in pan-canceris analyzed by big bioinformatics database. Chi-square test was used to analyze the relationship between hnRNPH1 expression level in glioma tissues and clinicopathological parameters. Univariate and multivariate regression analysis were used to analyze hnRNPH1 expression level and clinicopathological parameters for predicting survival of patients with glioma. Kaplan-Meier curve was used to analyze the relationship between hnRNPH1 expression level and survival of tumor patients.Results The Cancer Genome Atlas (TCGA) database showed that hnRNPH1 was generally highly expressed in the tumor tissues. Compared with adjacent normal tissues, hnRNPH1 had a higher expression level in glioblastoma tissues, and the expression level of hnRNPH1 gradually increased with the age of patients. Results showed that the expression level of hnRNPH1 was positively correlated with the grade of glioma. Survival analysis showed that patients with high hnRNPH1 expression had a short overall survival. hnRNPH1 expression was not correlated to patient age, sex and tumor location ( P>0.05), while significantly correlated to tumor type and grade ( P<0.05). hnRNPH1 expression level, patient age and tumor grade were independent predictors of prognosis ( P>0.05).Conclusions The expression level of hnRNPH1 in glioma tissue was significantly up-regulated, which was closely related to the poor prognosis of tumor patients and may be involved in the occurrence and development of glioma.
胶质瘤是成年人颅内最常见的原发性恶性肿瘤, 根据其恶性程度, 胶质瘤可分为WHOⅠ ~Ⅳ 级, 其中WHO Ⅳ 级为恶性程度最高的胶质母细胞瘤。胶质瘤因其肿瘤异质性、弥漫性生长的特征导致患者的治疗效果及预后较差[1]。胶质瘤的规范化治疗包括手术切除、替莫唑胺辅助化疗加放疗或者替莫唑胺单纯化疗。恶性胶质瘤患者的中位生存时间只有约12~16个月[2]。目前胶质瘤的研究主要着眼于基因异常表达、基因突变及表观遗传学改变能否成为其诊断及预后的分子标志物。对胶质瘤的分子生物学进程进行更深入的研究, 充分利用新的分子靶点及新的技术提高胶质瘤的诊治水平, 具有非常深远的意义[3]。异质性胞核核糖核蛋白H1(heterogeneous nuclear ribonucleoprotein H1, hnRNPH1)是异质核糖核蛋白(hnRNP)家族的一员, 能与异质核RNA(hnRNA)相结合[4]。既往研究显示, hnRNPH1具有多个与其细胞定位密切相关的功能结构域, 与运输蛋白1(transport protein 1, TNPO1)结合后能从细胞质转运到细胞核内。hnRNPH1是横纹肌肉瘤细胞增殖和存活所需的蛋白[5]。然而, 目前hnRNPH1在胶质瘤中的表达情况以及其与临床病理关系较少有人研究, 因此, 本研究将通过检测hnRNPH1在胶质瘤组织中的表达情况, 以期为进一步研究胶质瘤治疗, 预后提供有价值的线索。
选取2000年1月至2012年12月于广东省人民医院诊治进行手术的胶质瘤患者311例, 生存时间随访至2020年6月。取胶质瘤患者手术切除并进行石蜡包埋的肿瘤组织及对应的癌旁正常组织制作成组织芯片, 手术样本离体后浸泡于10%福尔马林内, 后续行常规固定和石蜡包埋。年龄分布为2~78岁, 年龄的平均数和中位数均是42岁, 年龄> 42岁有160例, ≤ 42岁有151例。男性患者182例, 女性患者129例。WHO分期以2020年世界卫生组织中枢神经系统肿瘤分类为标准[6], 分为WHOⅠ 级15例, WHOⅡ 级82例, WHO Ⅲ 级86例, WHO Ⅳ 级128例。收集胶质瘤患者的一般资料:年龄, 性别, 肿瘤定位, 肿瘤临床分期, 肿瘤亚型。
纳入标准包括:病理确诊为胶质瘤; 通过本院伦理委员会审核(伦理号:KY2020-092-01), 符合相关伦理学规定; 所有研究对象及其家属知情, 并签署知情同意书。
排除标准包括:合并其他肿瘤、严重肝肾功能障碍、全身性感染、免疫系统疾病、精神疾病、严重代谢性疾病者; 术前接受过局部放疗、全身化疗、生物免疫治疗、其他辅助治疗或其他抗肿瘤治疗; 妊娠哺乳期女性; 资料不完整或者不真实者。
hnRNPH1抗体(货号:ab10374)购买自广州新晋生物有限公司。DAB染色液(货号:ZLI-9019)购买自广州永津生物科技有限公司。抗原修复液(货号:C1034-100ml)购买自广州剪刀手基因科技有限公司。生物信息学数据库(http://ualcan.path.uab.edu)。
免疫组织化学方法(immunohistochemictry, IHC)检测方法如下操作:脱蜡和水化:将已经制作完毕的胶质瘤组织芯片放置于65℃恒温培养箱中烘烤2 h进行脱蜡处理; 芯片置于二甲苯中浸泡10 min, 更换二甲苯后继续浸泡10 min, 重复3次。梯度乙醇水化:无水乙醇、95%乙醇、90%乙醇、80%乙醇、70%乙醇、60%乙醇、去离子水, 按顺序依次各浸泡5 min。
过氧化氢处理:用3%过氧化氢浸泡15 min, 去除内源性过氧化物酶影响。抗原修复:高压修复, 将EDTA(PH 8.0)溶解在去离子水中, 加热至开始沸腾, 组织芯片, 盖上不锈钢锅盖, 缓慢加压, 待高压气阀开始喷气时计时浸泡2 min, 缓慢降压, 待芯片自然冷却。封闭:在芯片上滴加正常山羊血清封闭液, 室温封闭40 min, 甩去多余液体。抗体孵育:滴加抗体, 4℃环境下孵育过夜。磷酸盐吐温缓冲液(phosphate buffered saline Tween-20, PBST)洗涤:4℃过夜后用PBST洗涤3次, 每次10 min, 二抗孵育:滴加二抗, 37℃恒温箱孵育1 h。PBST洗涤:二抗孵育后用PBST洗涤3次, 每次10 min。DAB染色:配置DAB染色工作液, 染色5~10 min, 在显微镜下掌握染色程度。苏木素:染色3 min, 流水冲洗3 h。盐酸酒精分化。干燥, 二甲苯通透, 封片镜检。
联合阳性细胞百分比以及细胞着色强度进行评分, 着色强度无色为0分, 淡黄色为1分, 棕黄色为2分, 棕褐色为3分。公式为:阳性细胞百分比× 细胞着色强度分值[7]。hnRNPH1免疫组化染色平均数为230分, 中位数为270分, 以中位数为cut-off值将胶质瘤病人分为hnRNPH1高表达以及低表达两组。
利用统计学软件SPSS 24.0进行统计学分析。计数资料均以(n, %)表示, 组间比较行卡方检验, 计量资料均以均数 ± 标准差(x ± s)描述, 组间比较用t检验, COX回归分析hnRNPH1表达量和临床病理参数对胶质瘤患者总生存的预测作用。Kaplan-Meier曲线法分析hnRNPH1表达水平与肿瘤患者生存期的关系。当P< 0.05时, 差异具有统计学意义。
利用生物信息学数据库检测hnRNPH1在泛癌组织中的表达量, 结果提示hnRNPH1在泛癌组织中普遍呈现高表达, 见图1。与正常组织相比, hnRNPH1在胶质母细胞瘤组织中有更高的表达水平(P=0.029), 而且随着肿瘤患者年龄增加, 其表达水平也逐渐上升, P< 0.05, 见图2。
 | 图1 大数据生物信息学分析hnRNPH1在泛癌中的表达水平Fig.1 The expression level of hnRNPH1 in pan-cancer was analyzed by big data bioinformatics |
| |
Note: A. The expression level of hnRNPH1 in glioblastoma tissues is higher than that in normal adjacent tissues; B. hnRNPH1 expression increased with the age of glioblastoma patients'> | 图2 大数据生物信息学分析hnRNPH1在不同组织和不同年龄的胶质母细胞瘤肿瘤患者中的表达量 注:A. 对比癌旁正常组织, hnRNPH1在胶质母细胞瘤组织中具有更高表达量; B. hnRNPH1表达量随着胶质母细胞瘤患者年龄增加而提高Fig.2 The expression of hnRNPH1 in GBM based on sample types and patient's ages were analyzed by big data bioinformatics Note: A. The expression level of hnRNPH1 in glioblastoma tissues is higher than that in normal adjacent tissues; B. hnRNPH1 expression increased with the age of glioblastoma patients |
组织芯片包含311例各级别胶质瘤及配对的癌旁正常组织, 免疫组化结果显示, 对比低级别胶质瘤, hnRNPH1蛋白表达在高级别胶质瘤中表达更高水平, 见图3。统计分析显示, hnRNPH1在WHO Ⅲ 级与WHO Ⅳ 级的高级别胶质瘤中具有更高水平表达量, 见图4A, 胶质瘤亚型表达分析也证实肿瘤级别越高, hnRNPH1表达水平也越高, hnRNPH1表达水平与肿瘤级别呈正相关, 见图4B。
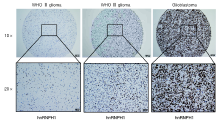 | 图3 免疫组化检测hnRNPH1在各级别胶质瘤中的表达水平Fig.3 The expression level of hnRNPH1 in different grade glioma was detected by immunohistochemistry |
利用组织芯片染色结果进行生存分析发现, hnRNPH1高表达的肿瘤患者预后更差, P=0.01, 见图5A。采用分层生存分析, 分别比较低级别胶质瘤及高级别胶质瘤亚组, hnRNPH1高表达的肿瘤患者均提示具有更差的预后, P< 0.000 1, 见图5B。进一步在数据库中进行分析, 同样发现hnRNPH1表达水平越高, 预后更差, P=0.007, P< 0.000 1, 见图5C、5D。
本研究纳入病例中, WHOⅠ 级15例, WHOⅡ 级82例, WHO Ⅲ 级86例, WHO Ⅳ 级128例。依据hnRNPH1的表达水平进行分组, 并进行卡方检验, 分析结果显示, hnRNPH1的表达水平与胶质瘤分级存在显著相关性, P=0.01; 而与肿瘤患者的年龄, 性别和肿瘤所在位置无关, P> 0.05, 见表1。
| 表1 免疫组化分析311例胶质瘤石蜡组织中hnRNPH1表达情况及其与临床病理参数之间的关系 Tab.1 Correlation of hnRNPH1 and clinicopathological parameters in the 311 glioma paraffin-embedded tissues based on IHC |
进一步对hnRNPH1以及临床病理参数分别进行单因素和多因素回归分析, 统计结果显示, 单因素还是多因素回归分析均显示hnRNPH1表达量(P=0.038)、肿瘤患者性别(P=0.002)及WHO分级(P=0.012)为胶质瘤患者预后的独立预测因子, 而肿瘤患者年龄、肿瘤亚型和肿瘤所处位置则对肿瘤患者预后无预测意义, 见表2。
| 表2 COX回归进行单因素和多因素分析hnRNPH1和其他临床病理参数对胶质瘤患者总生存的影响 Tab.2 Univariate and multivariate analyses of clinicopathological parameters and hnRNPH1 for overall survival in the 311 glioma paraffin-embedded tissues based on IHC |
胶质瘤尤其是胶质母细胞瘤是致死性较高的肿瘤[8], 胶质瘤与众多肿瘤相似, 其致病过程是多步骤且累积性的结果[9]。胶质瘤的发生发展主要与癌基因的激活如丝氨酸/苏氨酸蛋白激酶(BRAF)、磷酸肌醇3-激酶(phosphoinositide 3-kinase, PI3K)异构体、RAS及表皮生长因子受体(epidermal growth factor receptor, EGFR)等以及抑癌基因的失活如TP53、RB及哺乳动物雷帕霉素靶向基因(mammalian target of rapamycin, mTOR)等[10]。TP53及RB基因的失活亦可导致生长抑制逃逸, 这两个基因可修复基因损伤或将细胞周期的G1期转换成S期促进细胞调亡[11, 12, 13]。hnRNP是RNA结合蛋白的一大家族, 主要参与核酸代谢的多个步骤, 包括RNA剪接、mRNA稳定、转录及翻译的调节等。根据细胞内定位不同, hnRNP的亚基行使相应不同的功能。Shi等学者研究发现敲降hnRNP A2/B1可通过PI3K/Akt通路抑制宫颈癌细胞的增殖及侵袭能力[14]。Deng等发现hnRNP A2/B1可促进胶质瘤细胞增殖、侵袭及化疗(替莫唑胺)耐受等, 并诱导胶质瘤细胞凋亡及反应性氧化过激(reactive oxidative stress, ROS)的产生[15]。剪接因子在肿瘤中常过表达, 发挥类似癌基因的作用[16], hnRNPH1只与连续鸟嘌呤(G-tracts)结合, hnRNPH1对肿瘤相关基因进行选择性剪接, 从而影响患者预后[17, 18, 19]。过表达hnRNPH1通过调节肿瘤剪接调节影响其对凋亡的抵抗从而影响干细胞表达而发挥促进肿瘤的作用[20]。有研究表明hnRNPH1在胶质瘤中过表达且与肿瘤级别相关, 并可通过剪接调节促进肿瘤增殖及侵袭[16]。本研究发现hnRNPH1在胶质瘤中高表达, 且与肿瘤分级正相关, 提示hnRNPH1参与胶质瘤的发生发展并发挥着癌基因的作用。结合目前文献, hnRNPH1可能亦是通过调节胶质瘤的剪接因子从而发挥着癌基因的作用。其具体机制有待于我们进一步的研究。
进一步分析hnRNPH1在胶质瘤患者中的预后意义, 结果显示无论是单因素还是多因素分析, 均发现hnRNPH1在胶质瘤中具有独立的预后意义。hnRNP是肺腺癌、肝细胞癌等肿瘤预后不良的因素同时预示透明细胞性肾细胞癌及胸腺瘤较好的预后[21]。因此, hnRNP在肿瘤的发生发展及治疗、预后预测中具有不可忽视的作用。此前, Hope等学者的研究亦显示hnRNPH1与结直肠癌患者的预后密切相关[18]。目前, 暂未关于hnRNPH1在胶质瘤中预后预测中价值的研究, 本研究发现hnRNPH1是胶质瘤的独立预后不良因素, 提示hnRNPH1在胶质瘤的预后预测及治疗中具有潜在的价值。
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|