作者简介:
卢红莲(1995-),女,四川华蓥人,硕士研究生,主要研究方向为EGFR突变非小细胞肺癌。
目的 分析表皮生长因子受体(epidermal growth factor receptor, EGFR)非第18-21四个外显子(exons-18-21,Ex18-21)突变晚期非小细胞肺癌(non-small cell lung cancer,NSCLC)患者对靶向治疗、免疫治疗、化疗的疗效。方法 回顾性收集2016-2020年广东省肺癌研究所二代测序(next generation sequencing,NGS)数据库NGS检测出 EGFR非Ex18-21单突变肺癌患者35例, EGFR非Ex18-21复合突变肺癌患者65例,2016-2019年广东省肺癌研究所检测出 EGFR Ex18-21突变肺癌患者567例。同时收集3组患者的临床病理及治疗数据,分析3组患者的临床病理特征及 EGFR非Ex18-21突变肺癌对不同药物的治疗疗效。结果 EGFR非Ex18-21复合突变组患者与 EGFR Ex18-21组患者在年龄、性别、吸烟史、病理类型、TNM分期上均无统计学差异,而与 EGFR非Ex18-21单突变组患者在性别( P<0.001)、吸烟史( P<0.001)、病理类型( P<0.001)分布上有显著差异。 EGFR非Ex18-21复合突变组中接受第一代或第二代EGFR酪氨酸激酶抑制剂(tyrosine kinase inhibitors,TKI)患者( n=26)的中位无进展生存期(progression-free survival,PFS)为9.4个月,在倾向性评分匹配(propensity score matching,PSM)前后,都与 EGFR Ex18-21组的中位PFS无显著差异(PSM前: P=0.76;PSM后: P=0.76)。接受第三代EGFR-TKI( n=23)患者的中位PFS为9.5个月,在PSM前后,都与 EGFR Ex18-21组的中位PFS无显著差异(PSM前: P=0.23;PSM后: P=0.19)。 EGFR非Ex18-21单突变组中,接受免疫治疗患者的中位PFS为4.2个月,接受化疗患者的中位PFS为5.4个月。结论 在NGS检出突变后, EGFR非Ex18-21复合突变患者仍能从EGFR-TKI治疗中获益, EGFR非Ex18-21单突变患者接受免疫治疗和化疗的疗效与 EGFR野生型患者的疗效相似,未来需要探索EGFR-TKI在 EGFR非Ex18-21突变晚期NSCLC患者中的疗效。
Objective To analyze the response of epidermal growth factor receptor ( EGFR) non-exons-18-21-mutated advanced non-small cell lung cancer (NSCLC) to targeted therapy, immunotherapy, and chemotherapy. Methods This study collected the clinical data of 35 patients with EGFR non-exons-18-21 pure mutation and 65 patients with EGFR non-exons-18-21 compound mutation in the next generation sequencing (NGS) database of Guangdong Lung Cancer Institute (GLCI) from 2016 to 2020, and collected the clinical data of 567 patients with EGFR exons-18-21 mutation in GLCI from 2016 to 2019. Clinicopathological characteristics of the three groups were compared, and the response of patients with EGFR non-exons-18-21 mutations to different treatments were analyzed. Results The clinicopathological characteristics of the EGFR non-exons-18-21 compound mutation group were consistent with those of the EGFR exons-18-21 mutation group, but showed significant differences in gender ( P<0.001), smoking history ( P<0.001), and pathological type ( P<0.001) compared with those of the EGFR non-exons-18-21 pure mutation group. In the EGFR non-exons-18-21 compound mutation group, the progression-free survival (PFS) of patients receiving first- or second-generation EGFR-tyrosine kinase inhibitors (TKIs) ( n=26) was 9.4 months, which was no significant difference from that of patients with EGFR exons-18-21 mutation with or without propensity score matching (PSM) (before PSM: P=0.76; after PSM: P=0.76). And the PFS of patients receiving third-generation EGFR-TKIs ( n=23) was 9.5 months, which was also no significant difference from that of patients with EGFR exons-18-21 mutation with or without PSM (before PSM: P=0.23; after PSM: P=0.19). In EGFR non-exons-18-21 pure mutation group, the PFS of patients receiving immunotherapy and chemotherapy were 4.2 months and 5.4 months, respectively. Conclusions After NGS detected EGFR non-exons-18-21 mutation, patients with EGFR non-exons-18-21 compound mutations could also benefit from first- to third-generation EGFR-TKIs. And the response of immunotherapy and chemotherapy in patients with EGFR non-exons-18-21 pure mutations were similar to that of EGFR wild-type NSCLC. In the future, we need to explore the efficacy of EGFR-TKIs in EGFR non-exons-18-21 pure mutated NSCLC.
肺癌是全世界死亡率最高、发病率第二的癌症[1]。非小细胞肺癌(non-small cell lung cancer, NSCLC)约占原发性肺癌的80%~85%[2]。表皮生长因子受体(epidermal growth factor receptor, EGFR)基因是NSCLC最重要的驱动基因之一, EGFR突变主要集中于酪氨酸激酶域(tyrosine kinase domain, TKD), 尤其是第18-21四个外显子(exons-18-21, Ex18-21)。其中, EGFR第19外显子缺失(19 del)及第21外显子L858R突变是最常见的突变类型, 占85%~90%[3]。EGFR 第18外显子G719X, 第20外显子插入突变, 第21外显子L861Q等罕见突变约占10%[4, 5, 6]。第一代至第三代EGFR酪氨酸激酶抑制剂(tyrosine kinase inhibitors, TKI)显著延长了EGFR经典突变晚期NSCLC的无进展生存期(progression-free survival, PFS)[5, 7, 8, 9, 10, 11, 12], 第二代EGFR-TKI阿法替尼在治疗EGFR罕见突变晚期NSCLC也显示出显著的疗效[4, 13], 目前EGFR突变晚期NSCLC患者的5年生存率高达40%[14]。
随着二代测序(next generation sequencing, NGS)的广泛应用, EGFR非Ex18-21突变也常被检测出来。但这类突变发生率低, 目前对携带EGFR非Ex18-21突变NSCLC的临床病理特征及对不同药物的反应都尚不明确。
为分析EGFR非Ex18-21突变晚期NSCLC对不同治疗的反应, 我们回顾性收集广东省肺癌研究所EGFR非Ex18-21突变肺癌患者100例及EGFR Ex18-21突变患者567例。同时收集患者的临床病理特征及治疗数据, 并进行文献回顾。
回顾性收集2016-2020年广东省肺癌研究所NGS数据库中检测出EGFR非Ex18-21突变肺癌患者的临床数据, 和2016-2019年广东省肺癌研究所检测出EGFR Ex18-21突变肺癌患者的临床数据, 包括性别、出生日期、吸烟史、首次病理确诊肺癌时间、病理类型、TNM分期、转移部位、治疗过程等。
病理类型按照2021年世界卫生组织(World Health Organization, WHO)发布的第5版胸部肿瘤组织学分为腺癌、鳞癌、其他类型。疗效评估依据实体瘤疗效评估标准(Response Evaluation Criteria in Solid Tumors, RECIST)1.1版本, 完全缓解(complete response, CR)为靶病灶全部消失, 部分缓解(partial response, PR)为靶病灶直径之和缩小30%, 疾病稳定(stable disease, SD)为靶病灶直径之和缩小少于30%, 增大不超过20%, 疾病进展(progressive disease, PD)为靶病灶直径之和增大20%。PFS为开始用药至疾病进展的时间。
两组间连续变量比较采用t检验, 分类变量比较采用卡方检验或Fisher确切法检验。采用Kaplan-Meier方法和log-rank检验分析生存曲线, 为了增加可比性, 采用倾向性评分匹配(propensity score matching, PSM)平衡两组间差异, 匹配项目为年龄(≥ 65岁, < 65岁)、性别、吸烟史、病理类型、分期、治疗线数(1线, 2线, ≥ 3线), 匹配比率为1∶ 3, 匹配方法为最邻近匹配(即以倾向得分为依据, 在EGFR Ex18-21突变组样本中向前或向后寻找最接近EGFR非Ex18-21复合突变组样本得分的对象, 并形成配对), R语言基于Matching包进行PSM。使用R Studio软件及SPSS 22.0软件进行统计学分析, P< 0.05表示差异有统计学意义。
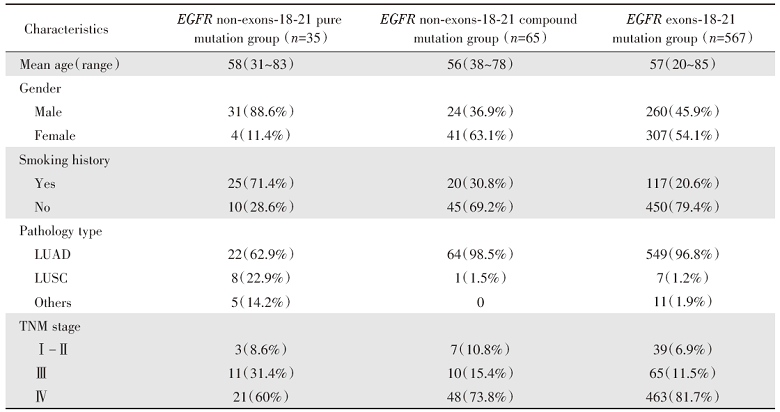
2016年2月至2020年11月广东省肺癌研究所总共有100例肺癌患者进行NGS后检测出EGFR非Ex18-21突变, 其中65例患者为EGFR Ex18-21合并EGFR非Ex18-21突变(EGFR非Ex18-21复合突变), 35例患者仅携带EGFR非Ex18-21突变(EGFR非Ex18-21单突变)。另外, 567例患者的基因检测提示EGFR Ex18-21突变。
EGFR非Ex18-21单突变组中, 88.6%为男性, 71.4%有吸烟史, 62.9%病理类型为腺癌, 22.9%为鳞癌。EGFR非Ex18-21复合突变组中, 63.1%为女性, 69.2%无吸烟史, 98.5%病理类型为腺癌。EGFR Ex18-21突变组中, 51.4%为女性, 79.4%无吸烟史, 96.8%病理类型为腺癌, 见表1。
| 表1 EGFR非Ex18-21突变患者及EGFREx18-21突变患者的临床病理特征 Tab.1 The clinicopathological characteristics of patients with EGFR non-exons-18-21 mutations and exons-18-21 mutations [n(%)] |
对三组患者的临床病理特征进行统计分析, EGFR非Ex18-21复合突变组与EGFR Ex18-21组患者在年龄、性别、吸烟史、病理类型、TNM分期上均无统计学差异。而EGFR非Ex18-21复合突变组与EGFR非Ex18-21单突变组患者在性别(P< 0.001)、吸烟史(P< 0.001)、病理类型(P< 0.001)分布上具有显著差异, 见图1。
剔除无效数据后(治疗数据缺失, 如开始用药时间、进展时间、是否进展等资料不详), EGFR非Ex18-21复合突变组有24例患者接受了第一代或第二代EGFR-TKI单药治疗, 总共26条靶向治疗数据(22例患者接受1次, 2例患者接受2次)。22例患者接受了第三代EGFR-TKI单药治疗, 总共23条治疗数据(21例患者接受1次靶向治疗, 1例患者接受2次靶向治疗)。
EGFR Ex18-21突变组有324例患者接受了第一代或第二代EGFR-TKI单药治疗, 总共352条靶向治疗数据(300例患者仅接受1次靶向治疗, 20例患者接受了2次靶向治疗, 4例患者接受了3次靶向治疗)。136例患者接受了第三代EGFR-TKI单药治疗, 总共143条靶向治疗数据(129例患者接受了1次靶向治疗, 7例患者接受了2次靶向治疗)。
EGFR Ex18-21突变组与EGFR非Ex18-21复合突变组接受第一代至第三代EGFR-TKI具体药物情况见图2。
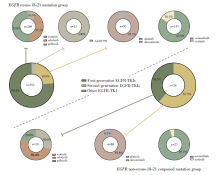 | 图2 EGFR Ex18-21突变组与EGFR非Ex18-21复合突变组靶向治疗药物的比例Fig.2 The proportion of targeted drugs in EGFR exons-18-21 mutation group and EGFR non-exons-18-21 compound mutation group |
2.2.1 第一代或第二代EGFR-TKI对EGFR非Ex18-21复合突变NSCLC的疗效分析
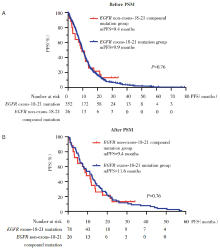
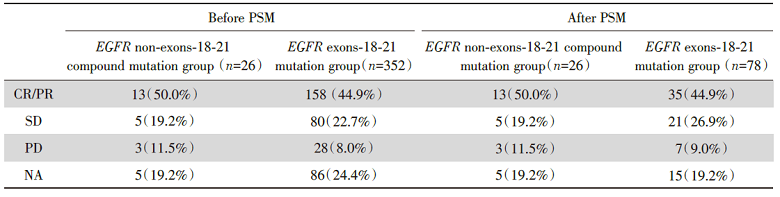
在PSM前, EGFR非Ex18-21复合突变组(n=26)患者的中位PFS为9.4个月, EGFR Ex18-21组(n=352)为9.9个月, 两组中位PFS无统计学差异, P=0.76, 风险比(hazard ratio, HR)[95%可信区间(confidence interval, CI)]0.93(0.61~1.43), 见图3A。
将年龄、性别、吸烟史、病理类型、TNM分期、治疗线数按照1∶ 3 PSM后, EGFR非Ex18-21复合突变组(n=26)患者的中位PFS为9.4个月, EGFR Ex18-21组(n=78)为11.6个月, 两组中位PFS无统计学差异, P=0.76, HR(95%CI)1.08(0.66~1.76), 见图3B。两组患者接受第一代或第二代EGFR-TKI治疗的具体疗效见表2。
| 表2 第一代或第二代EGFR-TKI在EGFR非Ex18-21复合突变及EGFR Ex18-21突变患者的疗效 Tab.2 The response of first- or second-generation EGFR-TKIs in patients withEGFRnon-exons-18-21 compound mutation and EGFR exons-18-21 mutation [n(%)] |
2.2.2 第三代EGFR-TKI对EGFR非Ex18-21复合突变NSCLC的疗效分析
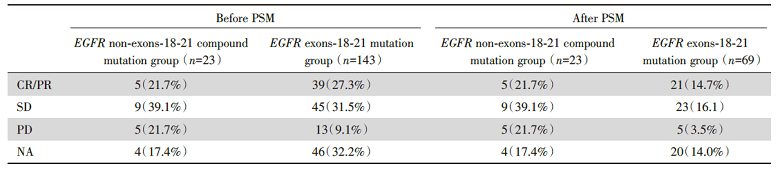
在PSM前, EGFR非Ex18-21复合突变组(n=23)患者的中位PFS为9.5个月, EGFR Ex18-21组(n=143)为8.2个月, 两组中位PFS无统计学差异, P=0.23, HR(95%CI)0.69(0.41~1.17), 见图4A。
将年龄、性别、吸烟史、病理类型、TNM分期、治疗线数按照1∶ 3 PSM后, EGFR非Ex18-21复合突变组(n=23)患者的中位PFS为9.5个月, EGFR Ex18-21组患者(n=69)为7.8个月, 两组的中位PFS无统计学差异, P=0.19, HR(95%CI)0.65(0.38~1.14), 见图4B。两组患者接受第三代EGFR-TKI治疗的具体疗效见表3。
| 表3 第三代EGFR-TKI在EGFR非Ex18-21复合突变及EGFR Ex18-21突变患者的疗效 Tab.3 The response of third-generation EGFR-TKI in patients withEGFR non-exons-18-21 compound mutation and EGFR exons-18-21 mutation [n(%)] |
在NGS检测出EGFR非Ex18-21单突变后, 35例患者中有21例患者接受了药物治疗。8例患者仅接受过免疫治疗(免疫单药或免疫联合化疗等), 6例患者相继接受过化疗(其中1例患者接受过两线化疗)和免疫治疗(免疫单药或免疫联合化疗等), 6例患者仅接受过化疗, 1例患者仅接受过抗血管单药治疗, 总共有28条治疗数据。
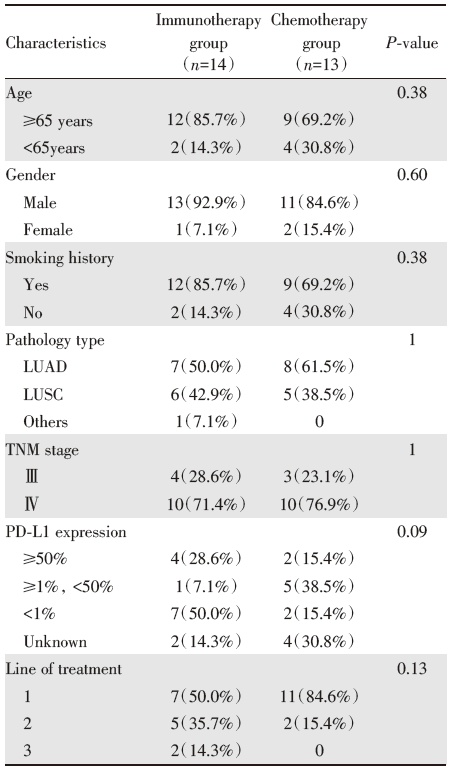
根据患者治疗情况, 将接受过免疫治疗的患者归为免疫治疗组(n=14), 接受过化疗的患者归为化疗组(n=13), 两组患者存在交叉。
两组患者在年龄、性别、吸烟史、病理类型(NGS检测出EGFR非Ex18-21突变后)、TNM分期(NGS检测出EGFR非Ex18-21突变后)、程序性细胞死亡蛋白配体-1(programmed cell death-ligand 1, PD-L1)表达率、治疗线数上都无统计学差异, 见表4。
| 表4 EGFR非Ex18-21单突变组接受免疫治疗和化疗患者的临床病理特征 Tab.4 The clinicopathological characteristics of patients receiving immunotherapy and chemotherapy inEGFR non-exons-18-21 pure mutation group [n(%)] |
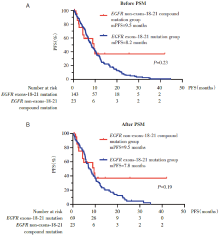
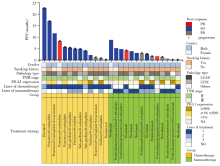
在EGFR非Ex18-21单突变患者中, 免疫治疗组的中位PFS为4.2个月, 化疗组的中位PFS为5.4个月(图5)。化疗组和免疫治疗组患者的最佳疗效, PD-L1表达等详细数据见图6。
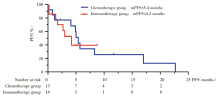 | 图5 免疫治疗和化疗在EGFR非Ex18-21单突变患者的生存分析Fig.5 PFS of patients receiving immunotherapy and chemotherapy in EGFR non-exons-18-21 pure mutation group |
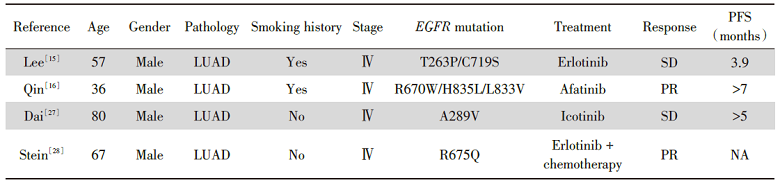
第一代至第三代EGFR-TKI已成为EGFR经典突变NSCLC的标准一线治疗药物, 阿法替尼也已批准用于EGFR罕见突变NSCLC的一线治疗。然而目前关于EGFR非Ex18-21突变NSCLC的治疗数据十分有限, 相关病例报道见表5。
| 表5 EGFR非Ex18-21突变NSCLC患者病例报道 Tab.5 Summary of case reports ofEGFR non-exons-18-21-mutated NSCLC |
一篇文章报道了一例NGS检测出EGFR T263P/C719S复合突变的NSCLC, 患者接受厄洛替尼治疗后, 疗效评估为SD, PFS只有3.9个月。但细胞实验结果显示, 阿法替尼能抑制携带EGFR T263P/C719S突变的Ba/F3细胞的生长, 表明这种复合突变对阿法替尼敏感[15]。另一文章报道了一例携带EGFR R670W/H835L/L833V复合突变的NSCLC患者, 阿法替尼为三线治疗, PFS超过7个月[16]。EGFR C719S、H835L、L833V都为EGFR罕见突变, 既往研究表明能从阿法替尼治疗中获益[4, 17]。2017年日本学者的研究[18]中发现, 携带R108K/A216T/A289T/V292L/S306L复合L858R或19del突变的细胞对吉非替尼、厄洛替尼、阿法替尼、奥希替尼敏感, A1118T复合19del突变对阿法替尼敏感。
本研究的结果表明, 在NGS检测出EGFR非Ex18-21复合突变后, 患者接受第一代或第二代以及第三代EGFR-TKI的疗效与EGFR Ex18-21突变患者无显著差异。接受第一代或第二代EGFR-TKI治疗的EGFR非Ex18-21复合突变患者中位PFS为9.6个月, 这也与既往临床试验报道的第一代或第二代EGFR-TKI的PFS数据相似[5, 7, 8, 9, 11]。这一结果的原因是EGFR非Ex18-21复合突变由于同时存在EGFR Ex18-21突变(绝大部分为L858R和EGFR 19del), 能使EGFR在缺失配体的情况下, 形成同源或异源二聚体, 激活TKD并发生自身磷酸化, 导致下游通路的激活, 引起肿瘤细胞的生长、增殖、分化、转移[19]。而第一代EGFR-TKI能与ATP竞争性结合EGFR的ATP结合位点, 第二代和三代EGFR-TKI与ATP结合袋边缘的797位点的半胱氨酸残基共价结合, 能抑制EGFR TKD的活化及CT区域的自身磷酸化, 阻碍下游信号通路, 达到抗肿瘤的目的[22, 24, 26]。
在靶向药物和免疫药物出现之前, 铂类为基础的化疗是肺癌的标准治疗, 晚期NSCLC患者的中位PFS大约4~6个月[20, 21, 22, 23]。本研究中, 接受化疗的EGFR非Ex18-21单突变患者中位PFS为5.2个月, 这一结果与既往研究结果相似。免疫检查点抑制剂问世后, 程序性死亡受体1(programmed death 1, PD-1)、PD-L1抑制剂相关的临床试验层出不穷, 致力于探索出精准的获益人群。CheckMate-017和CheckMate-057是两项针对一线化疗失败后, 探索纳武利尤单抗在晚期肺鳞癌和腺癌疗效的研究。接受免疫治疗的患者无PD-L1表达率的限制, 两个临床试验结果显示纳武利尤单抗在肺鳞癌和肺腺癌的中位PFS分别为3.5个月、2.3个月[24, 25]。KEYNOTE-189是一项探索帕博利珠单抗联合培美曲塞+铂类对比培美曲塞+铂类在一线非鳞状NSCLC的疗效的研究, 结果显示免疫联合化疗显著延长了患者的生存, 而培美曲塞联合铂类PFS仅4.9个月[26]。在本研究中, 接受免疫治疗的EGFR非Ex18-21单突变患者PD-L1表达情况不一, 且免疫治疗多为一、二线治疗, 中位PFS为4.2个月, 与这些研究结果相似。
根据NCCN指南, 由于EGFR Ex18-21突变阴性, EGFR非Ex18-21突变患者缺乏EGFR-TKI治疗的证据。经过PubMed检索发现, 一篇文章报道了EGFR A289V突变的患者能从第一代EGFR-TKI埃克替尼中获益, 治疗5个月后肿瘤缩小30%[27]。另一项研究报道, 一例EGFR R675Q突变多发骨转移患者在厄洛替尼联合紫杉醇、卡铂治疗两周期后达到PR[28]。似乎某些携带EGFR非Ex18-21突变的肺癌患者也能从EGFR-TKI治疗中获益, 因此, 未来需要探索EGFR-TKI对EGFR非Ex18-21突变的疗效。
在NGS检出突变后, EGFR非Ex18-21复合突变患者能从第一至第三代EGFR-TKI治疗中获益, EGFR非Ex18-21单突变患者接受免疫治疗和化疗的疗效与EGFR野生型患者的疗效相似, 未来需要探索EGFR-TKI在EGFR非Ex18-21突变晚期NSCLC患者中的疗效。
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|